Introduction: Sickle Cell Disease (SCD) is an inflammatory and vaso-occlusive pathology. Multiple lipid mediators: resolvins, protectins and maresins, collectively known as Specialized Pro-resolving Mediators (SPMs), that are biosynthesized from Omega-3-PolyUnsaturated Fatty Acids (O3PUFAs), docosahexaenoic acid and eicosapentaenoic acid, appear to play a role in the resolution of inflammation. In a recent study, we have documented the presence of SPMs including Resolvin-D1 (RvD1), 17R-RvD3, and their pathway markers in plasma from both children with SCD and age-and race-matched controls with a significant increase in RvD1 levels noted in plasma from children with SCD. In an SCD mouse model exposed to hypoxia/reoxygenation, a previous study has shown that oral administration of 17R-RvD1, the longer acting isomer of RvD1, promotes resolution of SCD-associated inflammation and tissue damage.
Objectives: We assessed whether there were any associations in children with SCD between baseline plasma levels of RvD1 and either markers of inflammation, endothelial activation and hemolysis or SCD-related clinical correlates.
Methods: Multiplex assays were employed to assess endothelial activation markers (soluble adhesion molecules VCAM-1, ICAM-1 and E-selectin), and inflammatory cytokines (IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP) in plasma. A 3-year retrospective review of individual SCD patient records was performed, and the number of painful episodes of vaso-occlusion or VOC, including those complicated by the occurrence of acute chest syndrome (ACS) were recorded. Total cumulative painful episodes over a 3-year period were included in the analysis. Spearman Rank Correlation was employed to assess the association between two variables.
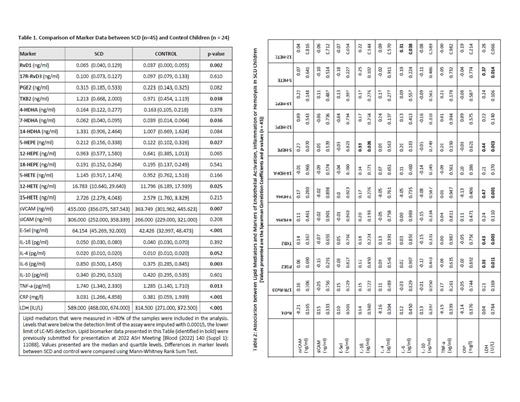
Results: Consistent with the published results, levels of endothelial activation markers (soluble VCAM-1 and soluble E-selectin), cytokines (IL-4, IL-6 and TNF-α), CRP and LDH were increased in SCD plasma compared to controls (Table-1). No significant associations were found in SCD between RvD1 or other lipid mediators and markers of endothelial activation or inflammation (Table-2). However, there were two significant associations noted between 5-HEPE and IL-1β, and between 12-HETE and IL-6. Plasma levels of the hemolytic marker LDH positively correlated with PGE2, TxB2, 7-HDHA, 5-HEPE and 5-HETE (Table-2). Review of medical history revealed 33 of 45 subjects (73%) had at least one VOC episode, and 17 (38%) had at least one ACS episode in the past 3 years. Subjects who had at least one ACS episode in the past 3 years also reported at least one VOC episode during the same period. There was no statistically significant difference in the RvD1 level by VOC [median RvD1 (ng/ml) of 0.056 in VOC -ve sub-group (n=12) vs 0.084 in VOC +ve sub-group (n=33), p=0.748] or ACS history [median RvD1 (ng/ml) of 0.060 in ACS -ve sub-group (n=28) vs 0.104 in ACS +ve sub-group (n=17), p=0.256]. There was no statistically significant association between levels of RvD1 and the total number of VOC/ACS in the past 3 years (correlation coefficient = -0.06, p=0.694).
Summary and Conclusions: Because of the documented pro-resolving and anti-inflammatory effects of orally administered 17R-RvD1 in a mouse model of SCD, and elevated basal plasma levels of RvD1 in children with SCD, we investigated whether endogenous RvD1 provides protection against inflammation and clinical complications in SCD children. Other lipid mediators, which were synthesized from O3PUFAs and arachidonic acid, present in increased levels in SCD plasma and potentially involved in modulation of inflammation were also included in the analysis. Unlike in SCD mouse model in which exogenous RvD1 exerted anti-inflammatory effect in ex-vivo experiments and orally administered 17R-RvD1 promoted resolution of inflammation and down-regulated adhesion molecule expression and cytokine production, endogenous RvD1 did not show any significant association with markers of inflammation and endothelial activation or clinical correlates in a small group of SCD children under healthy condition. No significant findings were noted with other lipid mediators. Whether these lipid mediators modulate inflammation during crisis, pre- or post-crisis clinical conditions and whether their production is different during these clinical conditions compared to steady state healthy condition require further investigation.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal